Saturation and Water Balance
Automatic Water Balance. Total Alkalinity, Calcium Hardness and Langelier Saturation Index
Langelier Saturation Index Total Alkalinity Calcium Hardness
Automatic Water Balance
As soon as a pool or spa is filled up with water – even before a sanitizer is added – one has to be concerned with water balance. The reason for it is that water is one of the most aggressive chemicals we have to deal with. If it does not contain enough key metals and minerals – in particular calcium – it will get them from its environment or if it contains too much of them, if will deposit them wherever possible. In other words, water has a definite affinity for certain inorganic compounds that must be satisfied at all times.
 From a chemical standpoint, water balance has to deal with inorganic compounds, such as calcium and carbonates that are soluble in water. Sanitation, on the other hand, deals with organic compounds, like algae, germs and bacteria that live in the water.
From a chemical standpoint, water balance has to deal with inorganic compounds, such as calcium and carbonates that are soluble in water. Sanitation, on the other hand, deals with organic compounds, like algae, germs and bacteria that live in the water.
The key factors in balancing pool or spa water in order of importance are:
- pH (ideal: 7.4 to 7.6)
- Temperature
- Total Alkalinity (ideal: 80 to 120 ppm)
- Calcium Hardness (ideal: 100 to 450 ppm)
- Total Dissolved Solids (TDS) (ideal: not above 2,000 ppm)
The CHEMTROL® Programmable Controllers range introduce automatic control of water balance by two methods:
- Control of Saturation by calculation of the Langelier Saturation Index to monitor to the development of scaling or corrosive conditions
- Monitoring and control of Conductivity to control the level of Total Dissolved Solids (TDS) by automatic replacement of contaminated water to reduce high TDS levels
Water Saturation
 The concern with water saturation results from the aggressive nature of dissolved minerals in water – particularly Calcium Carbonate – which can lead to scaling or etching of plaster and metallic equipment. It is a relatively new concept for pool and spa professionals and deserves to be thoroughly understood. In the past, water saturation was left to hazard and the results were that some pools would last forever while others would need constant repairs.
The concern with water saturation results from the aggressive nature of dissolved minerals in water – particularly Calcium Carbonate – which can lead to scaling or etching of plaster and metallic equipment. It is a relatively new concept for pool and spa professionals and deserves to be thoroughly understood. In the past, water saturation was left to hazard and the results were that some pools would last forever while others would need constant repairs.
Proper control of saturation and water balance is required to maintain water quality and to avoid the development of scaling or corrosive conditions. The Conductivity sensor monitors the concentration of dissolved solids by measuring the conductivity of the water. The data can be displayed either in conductivity units (micro-siemens/cm) or in parts per million (PPM) of Total Dissolved Solids (TDS), using an operator-selectable conversion factor.
 The CONDUCTIVITY MENU is used to set automatic dumping (bleeding) of water when the dissolved solid concentrations becomes too high, selecting either a Conductivity or a TDS control set-point to activate the dump valve. Backfilling of water is done simultaneously with a level control activated valve.
The CONDUCTIVITY MENU is used to set automatic dumping (bleeding) of water when the dissolved solid concentrations becomes too high, selecting either a Conductivity or a TDS control set-point to activate the dump valve. Backfilling of water is done simultaneously with a level control activated valve.
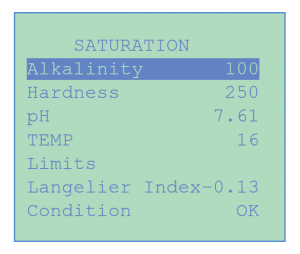
In order to facilitate the monitoring of water saturation conditions, the CHEMTROL® Programmable Controllers range automatically calculate and update the Langelier Saturation Index, taking sensor readings for pH and temperature and data entry for Alkalinity and Calcium Hardness.
Using standard Langelier Index limit values, the resulting water saturation condition is constantly displayed on the Main Screen as either “OK”, “Scaling” or “Corrosive”. If a scaling or corrosive condition develops, it is immediately indicated with a flashing display on the Main Screen.
“What if” analyses can be run at any time by the operator by manually entering different values for alkalinity, calcium hardness, pH and temperature. They are useful in predicting the effect of changes in the operating conditions.
The Langelier Saturation Index calculates the saturation condition from the pH and Temperature sensor inputs and from manual data entry for Alkalinity and Calcium Hardness. The water saturation condition is constantly displayed on the Main Screen as either “OK”, “Scaling” or “Corrosive” using standard Langelier Index limits. If a scaling or corrosive condition develops, it is immediately indicated with a flashing display on the Main Screen.
Studies in water treatment for boilers and cooling towers led to the development of the Langelier Saturation Index (SI) to determine the degree of saturation of Calcium Carbonate CaCO3 in water: SI = pH + TF + AF + CF -12.1.
Up to three different types of chemical additives – such as inhibitor and biocides for cooling towers – can be programmed separately for automatic addition as a function of time, bleed activation, pH control activation or cumulative flow rate.
The Langelier Saturation Index includes factors for pH, Total Alkalinity (AF), Calcium Hardness (CF), Temperature (TF) and to a lesser extent Total Dissolved Solids.
Manual calculation of this formula is rather cumbersome as it requires the use of separate data tables for each factor. The CHEMTROL® Programmable Controllers range do it automatically and accurately.
Total alkalinity (TA) is a concept that is not easy to explain because it is easily confused with acidity or basicity. In fact it refers to the buffering effect of dissolved compounds that resist changes in pH values.
TA is expressed in parts per million (ppm) and represents the concentration of alkaline compounds – such as bicarbonates, carbonates or hydroxides – which are dissolved in the water and oppose changes in pH.
Although there are many compounds capable of contributing to TA, it is normally expressed in ppm of “equivalent calcium carbonate”. For pools and spas, it should be maintained between 80 and 120 ppm.
The easiest way to test for TA is with test strips. Another method is with liquid test chemicals. First a pH indicator is added to stabilize the pH. Then an acid reagent is added until there is a color change. The more acid reagent is required, the higher the resistance to pH change and therefore the higher TA is.
High alkalinity increases the amount of acid required to adjust the pH. This is called high acid demand and leads to high pH and formation of bicarbonate scale.
Low alkalinity on the contrary causes unstable pH. This is referred to as pH bounce, which leads in turn to staining and increased corrosion.
LOWERING THE ALKALINITY
To lower the alkalinity: add 1 liter of muriatic acid or 1.1 Kg of granular acid per approx. 38,000 liter (40 m3) for each 12.5 ppm decrease.
RAISING THE ALKALINITY
To increase the alkalinity: add 700 g of Sodium Bicarbonate per approx. 38,000 liter (40 m3) of water for each 10 ppm increase.
Calcium Hardness describes the water quality referring to the minerals, mainly calcium and magnesium compounds, which may deposit scale in pipes, walls and heaters. Carbonate and bicarbonate hardness also adds to alkalinity. Sodium and potassium compounds do not contribute to hardness.
High hardness contributes to scaling but low hardness is also undesirable as it makes the water aggressive with regard to plaster, grouting between tiles. The recommended hardness for pool plaster protection is within 100 to 450 ppm depending on pH and Total Alkalinity.
To correct low hardness (under 100 ppm), add 450 g of Calcium Chloride per approx. 38,000 liter (40 m3) of water to raise it by 10 ppm. Do not apply through skimmer and add progressively in small quantities.
To correct high hardness (over 450 ppm), the water should be replaced or partially softened. Iron hardness can be precipitated by high chlorine residuals. A scale inhibitor or replacement of water can also be used. Tri-sodium phosphate can also be used to precipitate the calcium.
